The Permission to Contact (PTC) Platform
A patient enrollment strategy that facilitates patient engagement in research by inviting all patients to be participants in research.What is the PTC Platform?
The Permission to Contact (PTC) Platform overcomes the obstacles of consenting and patient enrollment by asking patients 'do you give permission to be contacted for future research opportunities?' as part of the routine health clinic practice. The Platform streamlines recruitment of participants enabling completion of research studies, especially for studies requiring a large number of participants.
The PTC Process
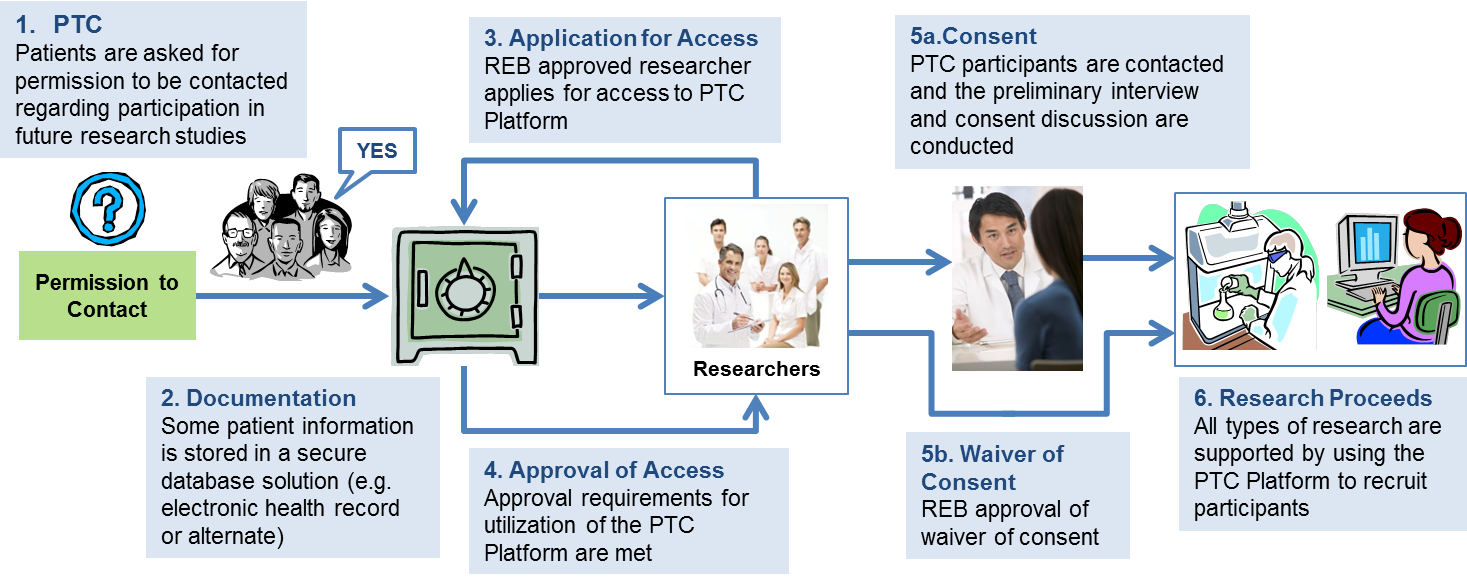
- Patients are presented with the opportunity to give their permission to be contacted for future research studies.
- Patient information is documented (for example within their electronic health record or in an alternate secure database solution)
- Researchers apply to ethics review boards to gain
- approval to access to the patient data and
- once approved utilize the PTC Platform.
- This approval may be subject to the requirement that researchers contact the patients for specific consent and the study design may necessitate approaching the patients for additional biospecimens or information or
- this approval may include a 'waiver of consent' for some study designs
- research proceeds.
Please read our published manuscript for more information:
Permission to Contact (PTC) - A Strategy to Enhance Patient Engagement in Translational Research.
How has the PTC Platform helped research participation?
Analysis of three example PTC programs shows that patients from diverse patient groups are agreeable to being approached about research (Cheah et al., 2013). The rates of acceptance (percentage of patients that gave permission to be contacted when asked) were:- over 93% of patients in the BC Cancer Agency Vancouver Island Centre program,
- over 80% at the Heart Centre clinics, and
- over 90% at the Women's pre-eclampsia clinic.
Why would I want to setup a PTC platform in my clinic or centre?
The PTC platform is applicable to enhancing patient enrollment across the spectrum of clinical research, and can benefit basic research, translational and clinical research studies, clinical trials, and all studies involving biobanking. PTC has been shown to have many benefits for different stakeholders in health research (e.g. patient and health staff engagement, centre and institutional oversight and management of patient recruitment into research, efficiency of enrollment for researchers). The main goal of the PTC platform at each site needs to be determined and can influence the design details to align with the priorities.
Please read our published manuscripts for more information:- Overcoming the translational
roadblocks: a cancer care and research model.
Braun L, Daudt HM, Watson P. Clin Transl Med. 2014 May 9;3:11. PMID: 24900890 - Impact of a 'Permission to Contact'
(PTC) Platform on Biobank Enrollment and Efficiency.
LeBlanc J, et al. Biopreservation and Biobanking. 2013; 11(3):144-149. PMID: 24850090. - Permission to Contact (PTC) - A
Strategy to Enhance Patient Engagement in Translational Research.
Cheah S, O'Donoghue S, et al. Biopreservation and Biobanking. 2013; 11(4):245-252. PMID: 24845592.
How long will it take and how much will it cost to implement?
The time and costs for implementation will depend on many variables including the type and scale of the PTC program. For all models, implementation of the PTC Platform requires committed support from the leaders of the institution or centre.
Costs will vary depending on the institution or centre operations and complexity of the PTC program.
Some start-up factors to consider are:Personnel
- A project manager at 0.5 FTE dedicated for approximately 6 months to guide program development, stakeholder engagement, and clinical personnel training
- Additional consulting services if required to support this individual (this is offered by the BRC, contact us for more information)
- IT Department assistance with selecting the best data management solution (e.g. addition to the electronic health record or creating/adapting an existing database for PTC, identifying server requirements).
- Additional consulting services if required to address database requirements (this is offered by the BRC, contact us for more information)
- Infrastructure
- Computer/equipment, office space
- Supplies
- Cost of staff training materials
I want to set up a PTC, what resources are available to me?
The PTC Tool Kit is now available!
Recently in partnership with Network of Networks (N2), we have developed a PTC Tool Kit to enable organizations across Canada to efficiently implement the PTC Platform, and to begin setting a national standard for the key elements of PTC. This tool kit has been developed to provide practical "how to" information and resources for individuals interested in setting up a PTC program within their organization. The in-depth downloadable PDF document contains information on budget considerations, stakeholder engagement, data management, and a strategy checklist.
The Tool Kit table of contents can be viewed here and the full Tool Kit is available for members of the BRC or N2.
If you are a member of N2, log in to N2 to access the Tool Kit. For information on how to become a member of N2, contact n2@n2canada.ca
If you are already a member of the BRC, Log In and download the PTC Tool Kit under Documentation Templates under My Dashboard.
Not a member? Sign Up as a new member of the BRC now!
We have also developed several templates and forms related to PTC which are available for download for Biobank Resource Centre members. See the list of available templates.
How can I get help to set up a PTC?
We can help! The Biobank Resource Centre offers consulting services for implementing the PTC Platform.
Services provided include:- Project management for implementation of the Platform
- Staff engagement and training
- Creation of standard operating procedures and forms
- Assistance with ethics application
For more information about PTC consulting services, please contact us.
Groups previously assisted by the Biobank Resource Centre for implementation of PTC Platform:- BC Cancer Agency Vancouver Island Centre
- BC Cancer Agency Centre for Southern Interior
- Vancouver Island Health Authority
- BC Children's and Women's Hospital
- Providence Health Care Research Institute Heart Centre
